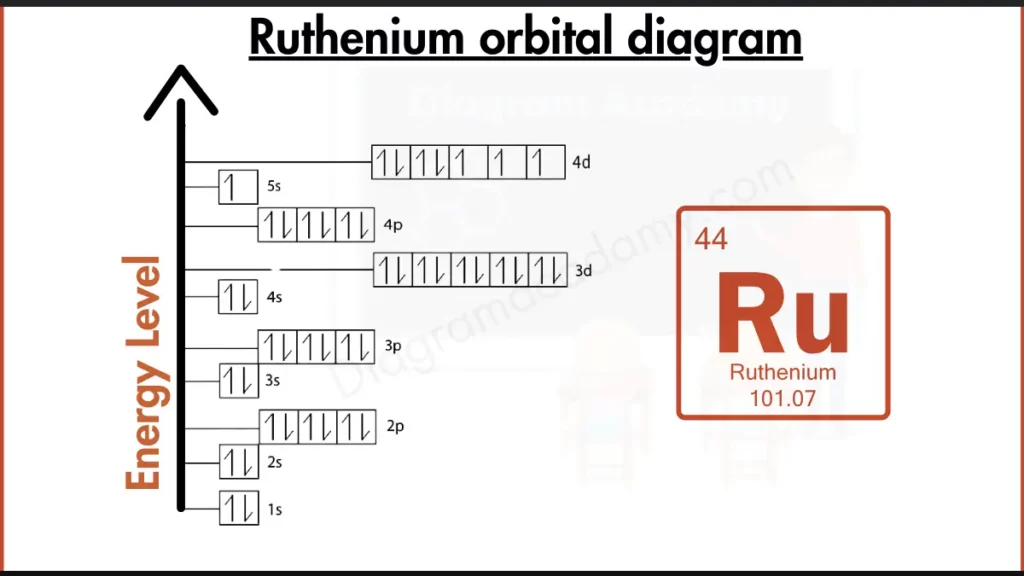
How to write the Orbital Diagram for Ruthenium?
Ruthenium defies the norm for transition metals. Instead of a completely filled 4d subshell, it has a stable configuration with seven electrons in 4d and one in 5s, making it more stable than some transition metals but still reactive compared to noble gases with their completely filled outer shells.