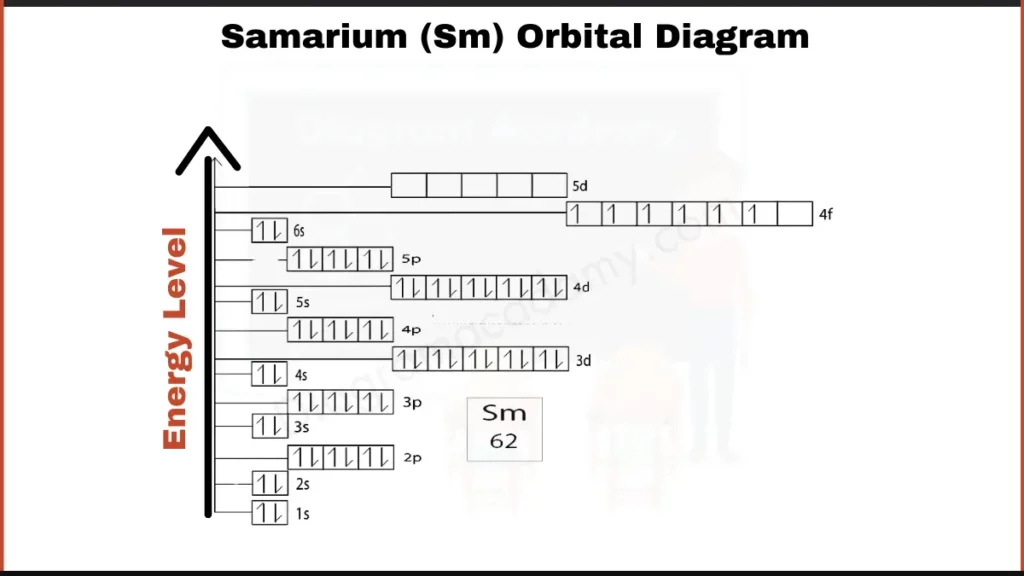
How to Write the Orbital Diagram for Samarium ?
Samarium (Sm) packs 62 electrons around its nucleus. These electrons fill shells in a specific order, but for a simpler picture, let’s focus on the outer ones. Like other lanthanides, Samarium has 6 electrons filling the 4f subshell and another 2 in the outermost shell, called 6s. These outer electrons play a key role in how Samarium interacts with other elements.