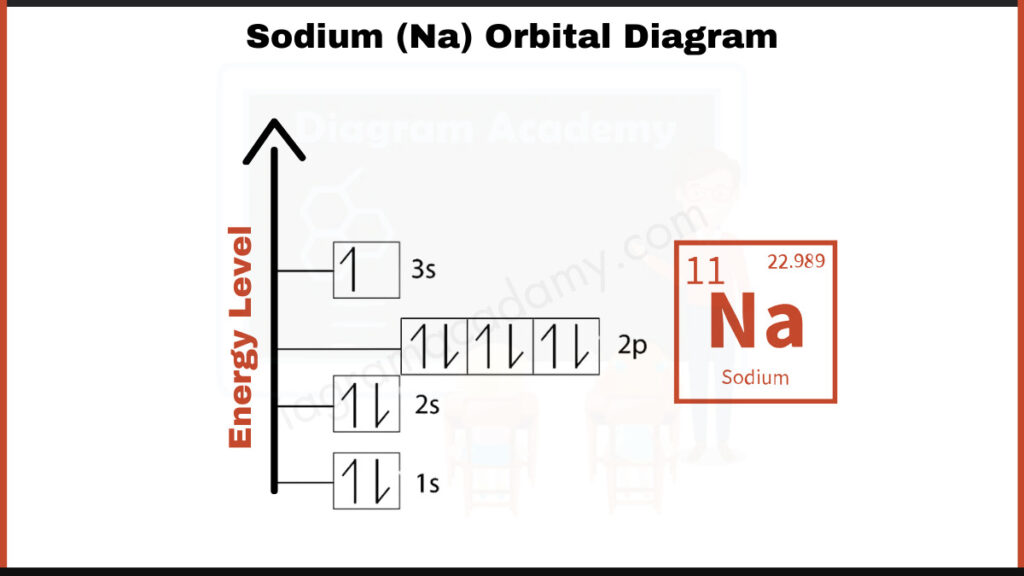
How to write the Orbital Diagram for Sodium?
Sodium (Na) has 11 electrons. They fill shells in a specific order: [2, 8, 1]. The first number, 2, represents the electrons in the innermost shell closest to the nucleus. The second number, 8, indicates the electrons in the second shell. Finally, the outer shell holds the remaining electron, represented by the number 1. This configuration helps chemists understand how sodium interacts with other elements during bonding.