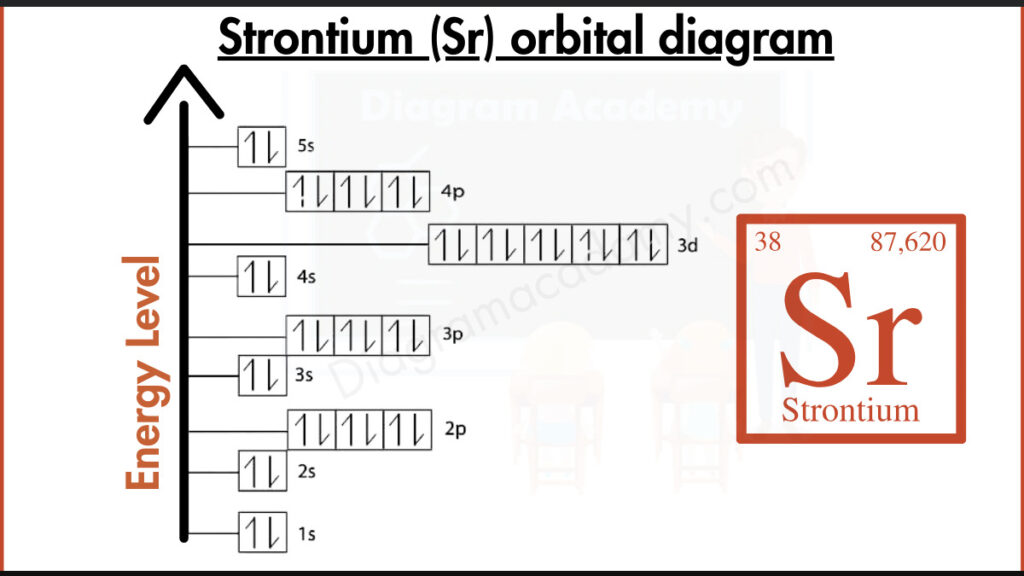
How to write the Orbital Diagram for Strontium?
Strontium (Sr) packs 38 electrons around its nucleus. The inner shells get filled first, like guests arriving at a party. Two electrons cozy up in the closest spot, followed by 8 in the next ring. Deeper inside, 18 electrons fill another shell. Finally, out on the dance floor, the outermost shell holds the stars of the show: 2 valence electrons that determine how strontium interacts with other elements.