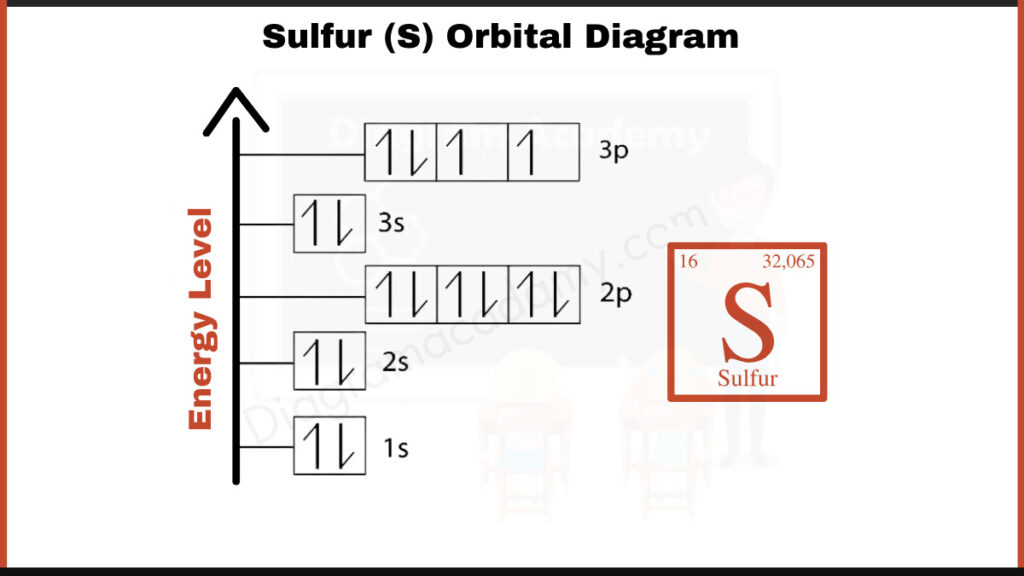
How to write the Orbital Diagram for Sulfur?
Sulfur (S) has 16 electrons. They fill shells in a specific order: [2, 8, 6]. This means the first shell (closest to the nucleus) holds 2 electrons, the second shell holds 8 electrons, and the third shell holds the remaining 6 electrons. This configuration can be used to depict the arrangement of electrons in Sulfur’s orbital diagram.