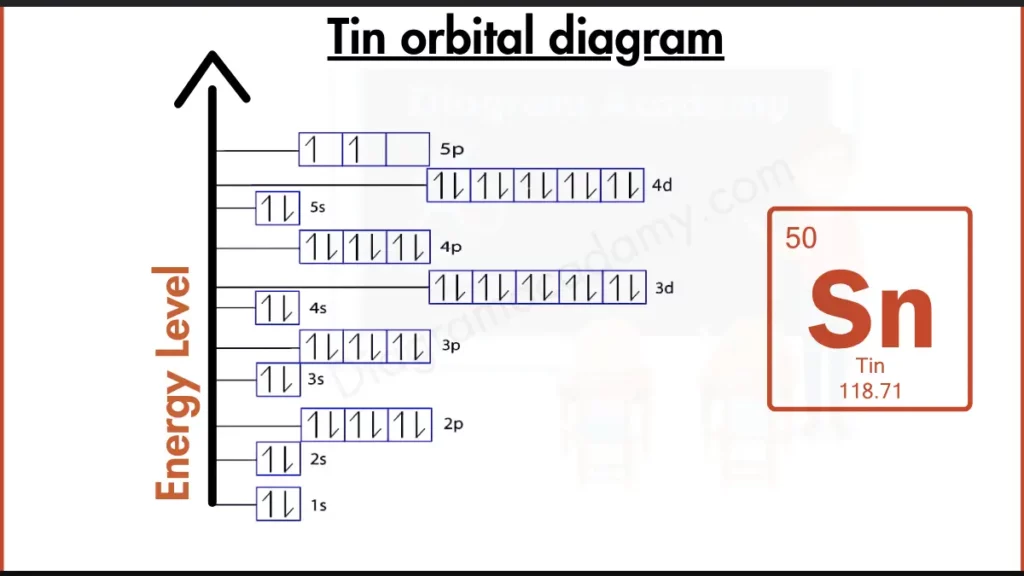
How to write the Orbital Diagram for Tin?
Tin (Sn) treads the line between reactive and stable. Its outer shell holds four electrons, configured as [Kr]4d¹⁰5s²5p². This arrangement showcases a filled 4d subshell and two electrons in the 5p subshell. While not completely full like the noble gases, Tin achieves a certain level of stability compared to elements with more vacancies in their outer orbitals. However, the presence of electrons in the 5p subshell still makes Tin somewhat reactive, especially compared to the completely inert noble gases