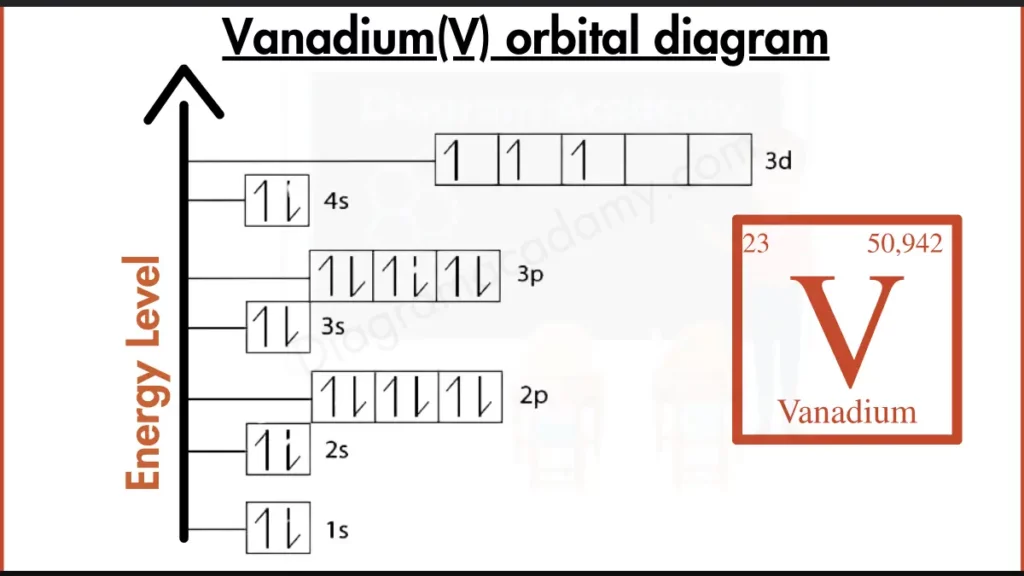
How to write the Orbital Diagram for Vanadium?
Vanadium (V) has 23 electrons filling shells around its nucleus. A common way to describe this is [2, 8, 13]. This notation indicates the number of electrons in each main shell. The first bracket, [2], represents the inner two shells that are completely filled with a total of 2 electrons. The second bracket, [8], represents the next shell containing 8 electrons. The final number, 13, signifies the electrons in the outermost shells of vanadium.