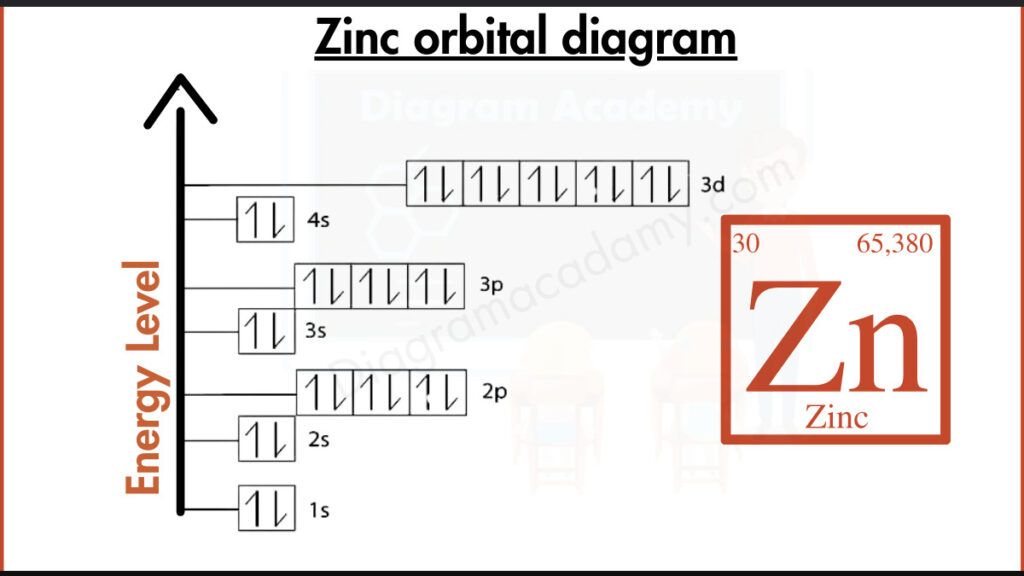
How to write the Orbital Diagram for Zinc?
Zinc crams 30 electrons around its core. The closest shell holds 2, the next 8, and a deeper shell stashes 18. Out on the frontier, the outermost shell holds the key players: 2 valence electrons responsible for zinc’s bonding with other elements.