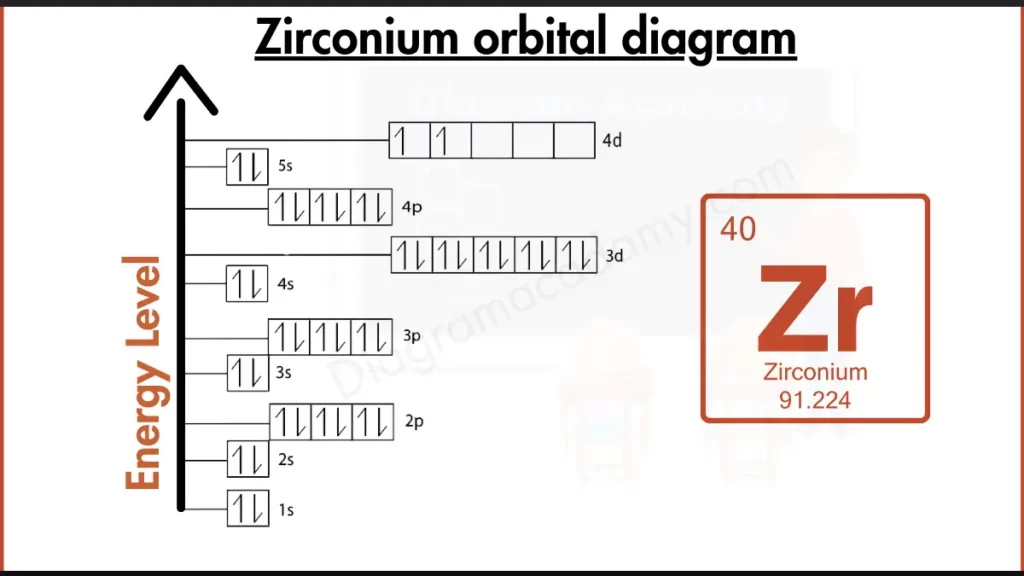
How to write the Orbital Diagram for Zirconium?
Zirconium (Zr) isn’t a party crasher, but it doesn’t quite join the noble gas club either. Its outer shell holds electrons in both the 4d and 5s subshells ([Kr] 4d² 5s²), leaving it incomplete. This partial filling, unlike the stable full shells of noble gases, makes Zirconium more reactive. However, it’s slightly more stable than some neighbors due to the filled inner shells represented by [Kr].